MRSI at ultra-high field
The ability to access information about the spatial distribution of metabolite concentrations within a single examination increases the value of in-vivo MR spectroscopy for diagnostics and physiological research. This is enabled by magnetic resonance spectroscopic imaging (MRSI), permitting the acquisition of spatially resolved spectra. Low SNR and long acquisition times at sufficient spatial resolution along with a strict limitation of the number of detectable metabolites at standard clinical field strength (1.5T) as well as imprecise and time-consuming quantification have prevented the technique from widespread adoption into clinical practice and physiological research in humans.
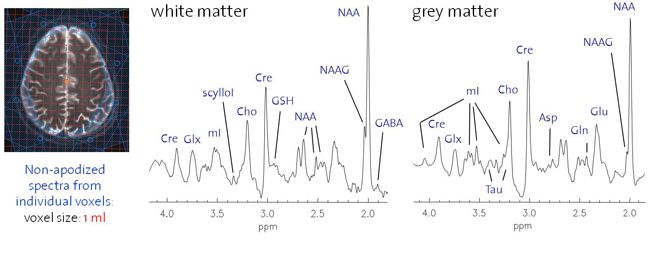
MRSI is one of the most promising applications of in-vivo high (3T) and ultra-high (7) field MR. Benefiting greatly from SNR gain and increased spectral resolution, highly spatially resolved detection and reliable quantification of an expanding number of metabolites becomes feasible. Reaping these benefits is particularly desirable for clinical brain and prostate MRSI aiming at spectral information from the entire organ including cortical brain tissue or prostate periphery. In addition, the possibility to map additional metabolites such as neurotransmitter or antioxidant distributions might give insight into the pathophysiology of neurological and psychiatric disorders and make MRS based clinical diagnostics more specific. However, high-field MRSI suffers from additional problems such as inappropriate shimming, localization, lipid and water suppression as well as B1 weighting and thus from a high artifact content.
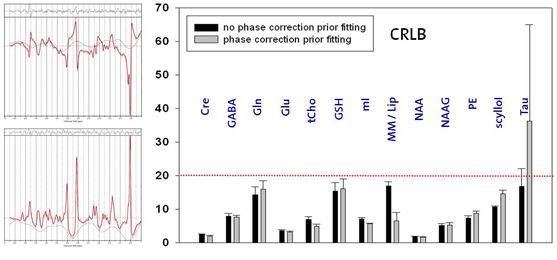
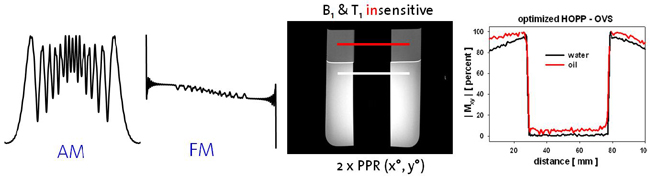
Thus, this ongoing project aims at the development of MRSI sequences suited for high and ultra-high fields (3T, 7T) in order to take advantage of the field dependent increase in spatial and spectral resolution. RF pulse design and the development of novel MRSI sequences should overcome localization problems such as the chemical shift displacement artifact which renders metabolite ratios inconsistent over the desired field-of-view. B1 and T1 insensitive water and lipid suppression techniques as well as robust coherence pathway selection method diminish the artifacts content. B1 inhomogeneities might be addressed by advanced RF concepts such as RF shimming or travelling wave excitation or B1 correction based on related B1 maps. Bo inhomogeneities might be improved by Bo mapping based shimming, dynamic shim update for multi-slice MRSI or additional passive or active shim elements. In addition, point spread functions might be tailored by highly spatially resolved MRSI enabled by various acceleration techniques such as sensitivity encoding, echo-planar spectroscopic imaging or partial fourier spectroscopic imaging. For sensitivity-encoded MRSI the maximal acceleration factor should increase substantially at 7T and above and needs to be determined. Last but not least absolute quantification for instance based on the ERETIC method or internal water referencing shall be enabled and robust fitting routines that include macromolecular baselines, a refined basis-metabolite set and spatial statistics need to be developed.
Publications:
- Henning A, Fuchs A, Murdoch JB, Boesiger P Slice-selective FID acquisition, localized by outer volume suppression (FIDLOVS) for 1H-MRSI of the human brain at 7T with minimal signal loss. NMR Biomed22(7), 683-696, 2009
- Henning A, Schär M, Schulte RF, Wilm B, Pruessmann KP, Boesiger P SELOVS: Brain MRSI localization based on highly selective T1- and B1-insensitive outer-volume suppression at 3T . Magn Reson Med59(1), 40-51, 2008
- Schulte RF, Henning A, Tsao J, Boesiger P, Pruessmann KP Design of broadband RF pulses with polynomial-phase response. J Magn Reson 186, 167-175, 2007
- Schulte R.F., Tsao J., Boesiger P., Pruessmann K.P. Equi-Ripple Design of Quadratic Phase RF Pulses. J Magn Reson 166, 111-122, 2004
- Sanchez-Gonzalez J, Tsao J, Dydak U, Desco M, Boesiger P, Paul Pruessmann K. Minimum-norm reconstruction for sensitivity-encoded magnetic resonance spectroscopic imaging. Magn Reson Med55(2), 287-95, 2006
- Dydak U., Weiger M., Pruessmann K.P., Meier D., Boesiger P. Sensitivity-Encoded Spectroscopic Imaging. Magn Reson Med 46, 713-722, 2001
Contact
No database information available